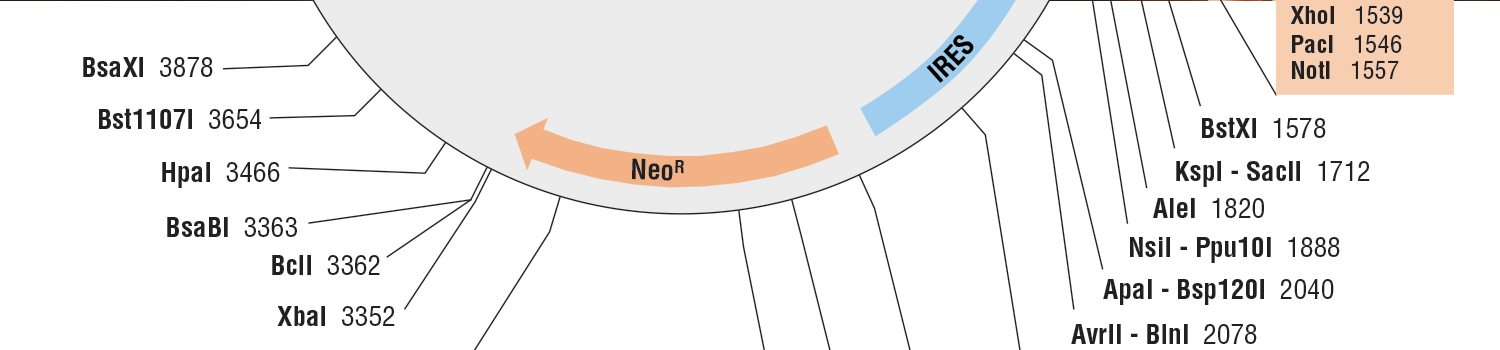
Protein Localization
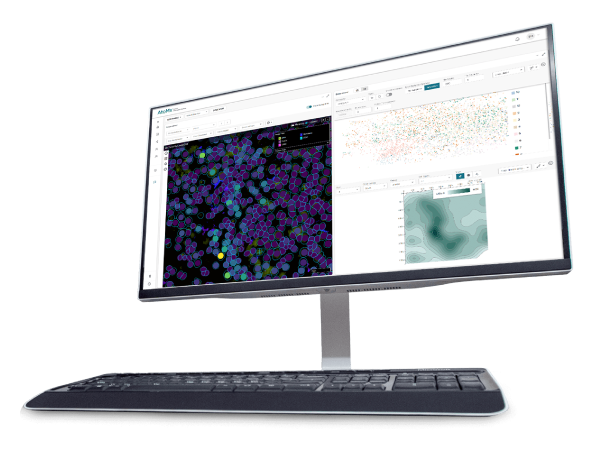
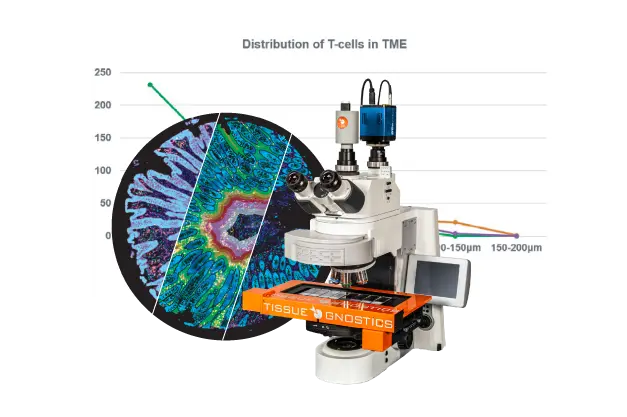
Protein functional activities correspond with their subcellular __expression__ and molecular complexing interactions. Localization can be effectively demonstrated with fluorescence microscopy based techniques or fractionation procedures. A broad spectrum of fluorescence imaging can be accomplished by using recombinant reporter proteins, (i.e. GFP, SNAP-tag®), or fluorescent dyes (i.e. Alexa), or fluorophore labeled molecules (i.e. protein specific antibodies). Cells can be genetically modified to overexpress protein targets, or regulatory components of non-coding sequences, for purposes such as determining fundamental cellular processes, disease mechanisms, gene therapy, and response to therapies. Fusion protein tags can be detected by antibodies or have functional properties to enable localization. Bioluminescent protein and fluorescent protein labeling systems are such genetically engineered optical imaging tools, with greater specificity at lower concentrations than other methods. With this approach, imaging can be in fixed or living cells, tissues or animals. A very attractive feature of the labeling of fusion proteins is that the labeling itself can be restricted to certain locations of a cell (i.e. SNAP-Cell® or SNAP-Surface®). Such discrimination cannot be easily achieved when using bioluminescent proteins. Some fluorescent protein labeling systems enable small nonfluorescent molecules to become fluorescent when bound to a small genetically inserted peptide sequence in the target protein of interest (i.e. FlAsH). Advances in genetically engineered fluorescence systems and microscopy optic machinery, have made imaging a core method for protein localization.
Feature
- 1
Clone and express once, then use with a variety of substrates
- 2
Non-toxic to living cells
- 3
Wide selection of fluorescent substrates
- 4
Highly specific covalent labeling
- 5
Simultaneous dual labeling
Product Information
Protein Localization
| Cat No. | SIze | |
|---|---|---|
| CLIP-Biotin | S9221S | 50 nmol |
| CLIP-Cell™ 505 | S9217S | 50 nmol |
| CLIP-Cell™ Block | S9220S | 100 nmol |
| CLIP-Cell™ TMR-Star | S9219S | 30 nmol |
| CLIP-Surface™ 488 | S9232S | 50 nmol |
| CLIP-Surface™ 547 | S9233S | 50 nmol |
| CLIP-Surface™ 647 | S9234S | 50 nmol |
| pCLIPf Vector | N9215S | 20 µg |
| pSNAPf Vector | N9183S | 20 µg |
| pSNAP-tag® (T7)-2 Vector | N9181S | 20 µg |
| SNAP-Biotin® | S9110S | 50 nmol |
| SNAP-Cell® 505-Star | S9103S | 50 nmol |
| SNAP-Cell® Block | S9106S | 100 nmol |
| SNAP-Cell® Oregon Green® | S9104S | 50 nmol |
| SNAP-Cell® TMR-Star | S9105S | 30 nmol |
| SNAP-Cell® 430 | S9109S | 50 nmol |
| SNAP-Cell® 647-SiR | S9102S | 30 nmol |
| SNAP-Surface® 488 | S9124S | 50 nmol |
| SNAP-Surface® 549 | S9112S | 50 nmol |
| SNAP-Surface® 594 | S9134S | 50 nmol |
| SNAP-Surface® 649 | S9594S | 50 nmol |
| SNAP-Surface® Alexa Fluor® 488 | S9129S | 50 nmol |
| SNAP-Surface® Alexa Fluor® 546 | S9132S | 50 nmol |
| SNAP-Surface® Alexa Fluor® 647 | S9136S | 50 nmol |
| SNAP-Surface® Block | S9143S | 200 nmol |