In vivo Imaging
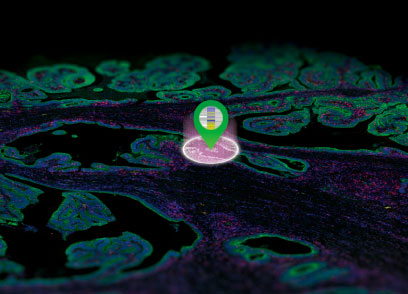
The dynamic real-time localization and translocation of molecules in living cells is an integral aspect of cellular function. Scientists are pioneering new applications for in vivo cellular and molecular imaging. These applications are based on genetic engineering advances and improved imaging technologies. In vivo imaging has become quantifiable, highly sensitive and amenable to high-throughput study design. The high sensitivity and utility of in vivo imaging, is exemplified by use of the Fluorescence Resonance Energy Transfer (FRET) method, which quantitates enzyme activity, protein-protein interactions and second messenger dynamics. Optical in vivo imaging methods are being applied in pre-clinical research studies. Real time in vivo imaging studies in whole animals have been used to track cell-based therapies, and monitor response to chemotherapies (1).
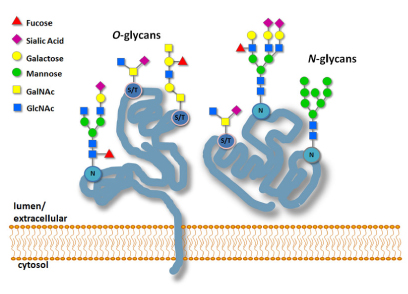
Fluorescence based recombinant systems are attractive molecular imaging tools because of their advantages for in vivo studies. Both bioluminescence reporters and fluorescence labeling systems offer relatively easy transition from in vitro, to living cells, tissues or small whole animal analysis (2). These protein labeling and reporter systems are non-invasive and allow direct analysis of cellular and molecular dynamics in real time. Some examples of bioluminescent reporters useful for in vivo imaging are enhanced GFP, FAP, Gaussia Luciferase (GLuc) and Cypridina Luciferase (Cluc). Protein labeling systems such as Tetra-Cys, SNAP-tag® and CLIP-tag™ can also be used for in vivo techniques. Imaging in living animals is currently best accomplished by PET reporters for scanning with short-lived radioactive tracers. Multimodality reporter gene systems have also been designed to permit in vivo imaging by multiple detection methods (3). For any fluorescence imaging application, fluorophore selection for native target activities and photostability should be confirmed in heterogeneous cellular environments.
Feature
- 1
Clone and express once, then use with a variety of substrates
- 2
Non-toxic to living cells
- 3
Wide selection of fluorescent substrates
- 4
Highly specific covalent labeling
- 5
Simultaneous dual labeling
Product Information
In vivo Imaging
| Cat No. | SIze | |
|---|---|---|
| CLIP-Biotin | S9221S | 50 nmol |
| CLIP-Cell™ 505 | S9217S | 50 nmol |
| CLIP-Cell™ Block | S9220S | 100 nmol |
| CLIP-Cell™ TMR-Star | S9219S | 30 nmol |
| CLIP-Surface™ 488 | S9232S | 50 nmol |
| CLIP-Surface™ 547 | S9233S | 50 nmol |
| CLIP-Surface™ 647 | S9234S | 50 nmol |
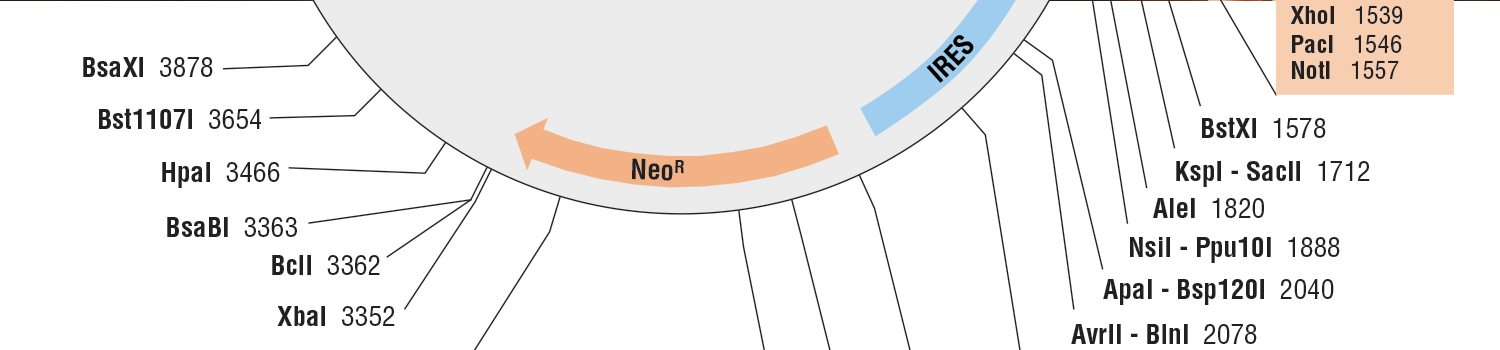
| pCLIPf Vector | N9215S | 20 µg |
| pSNAPf Vector | N9183S | 20 µg |
| pSNAP-tag® (T7)-2 Vector | N9181S | 20 µg |
| SNAP-Biotin® | S9110S | 50 nmol |
| SNAP-Cell® 505-Star | S9103S | 50 nmol |
| SNAP-Cell® Block | S9106S | 100 nmol |
| SNAP-Cell® Oregon Green® | S9104S | 50 nmol |
| SNAP-Cell® TMR-Star | S9105S | 30 nmol |
| SNAP-Cell® 430 | S9109S | 50 nmol |
| SNAP-Cell® 647-SiR | S9102S | 30 nmol |
| SNAP-Surface® 488 | S9124S | 50 nmol |
| SNAP-Surface® 549 | S9112S | 50 nmol |
| SNAP-Surface® 594 | S9134S | 50 nmol |
| SNAP-Surface® 649 | S9594S | 50 nmol |
| SNAP-Surface® Alexa Fluor® 488 | S9129S | 50 nmol |
| SNAP-Surface® Alexa Fluor® 546 | S9132S | 50 nmol |
| SNAP-Surface® Alexa Fluor® 647 | S9136S | 50 nmol |
| SNAP-Surface® Block | S9143S | 200 nmol |